Loss of functional neurons is a defining feature of neurodegenerative diseases and acute neural injuries. Because mature neurons lack the ability to self-replenish, their irreversible loss represents one of the greatest challenges in treating neurological disorders. For decades, the regeneration of functional neurons has been a central and elusive goal spanning academic research and pharmaceutical drug development.
Neural tissue is composed primarily of two cell types: neurons and surrounding supportive glial cells, including astrocytes. While neurons are post-mitotic and cannot regenerate, glial cells retain the capacity to divide and proliferate. Importantly, neurons and astrocytes share a common origin from neural stem cells and exhibit overlapping molecular and functional characteristics. Leveraging this intrinsic similarity, NeuExcell has developed an in situ ATN ASTROCYTES TO NEURONS TM platform that enables the direct conversion of glial cells into neurons within diseased or injured neural tissue.
At sites of neural injury, astrocytes are abundant, making them an ideal endogenous source for neuronal regeneration. By converting these resident glial cells into functional neurons, the ATN™ platform has the potential to generate substantial numbers of new neurons, repair damaged neural tissue, and restore lost neurological function. Preclinical studies have demonstrated robust efficacy in stroke and Alzheimer's disease animal models, including neural tissue repair, mitigation of motor and cognitive deficits, and significant extension of lifespan.
The ATN ASTROCYTES TO NEURONS TM platform supports the development of novel gene therapies across a broad range of neurodegenerative diseases. In 2021, NeuExcell Therapeutics entered into a strategic research collaboration with Spark Therapeutics, a member of the Roche Group, to advance gene therapies for neurological disorders, including Huntington's disease, using technology derived from the ATN™ platform. Building on strong preclinical validation, NeuExcell has advanced its neuroregeneration technology into the clinic, initiating a first-in-human clinical trial in patients with recurrent glioblastoma in 2024, followed by clinical trials in patients with Alzheimer's disease or stroke in 2025.
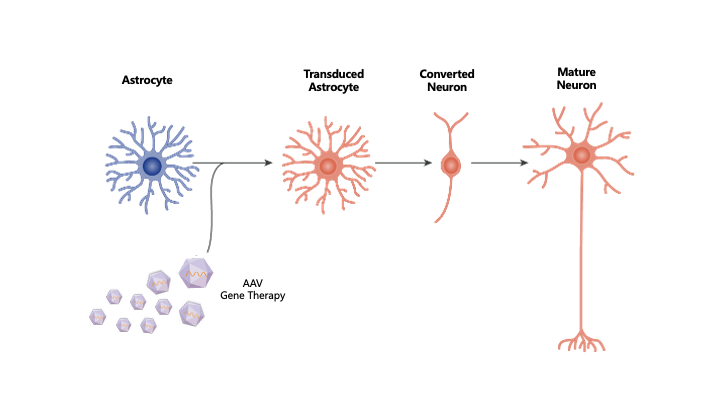
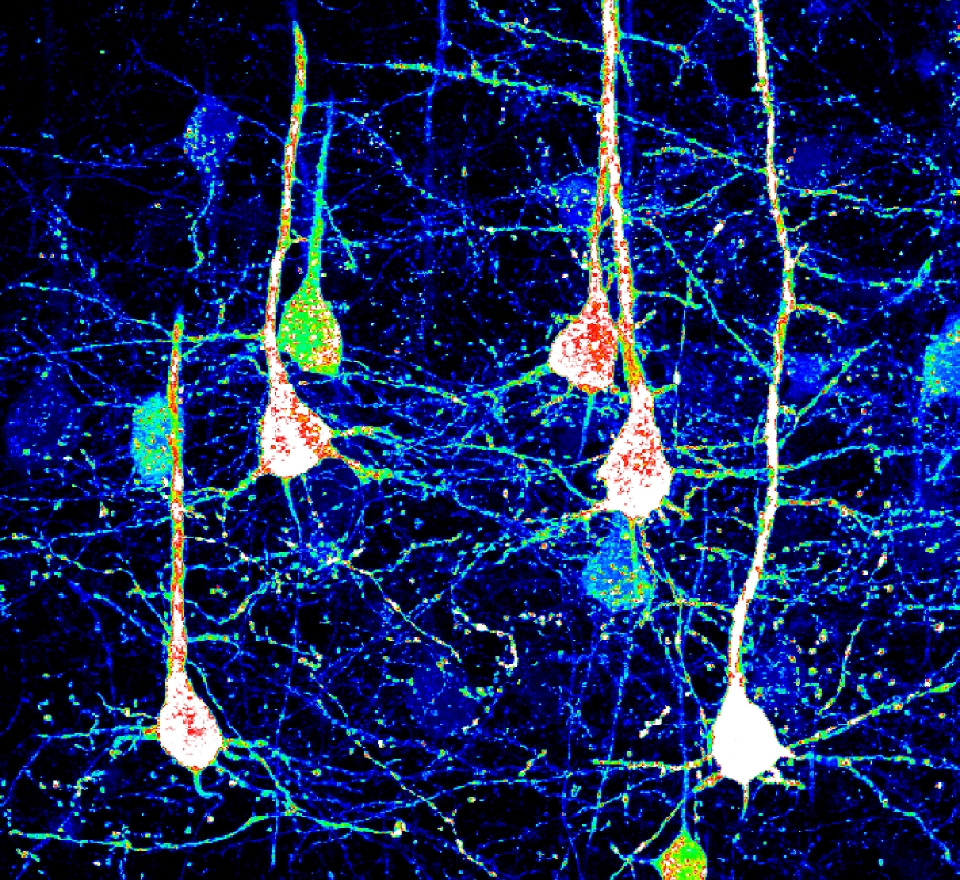
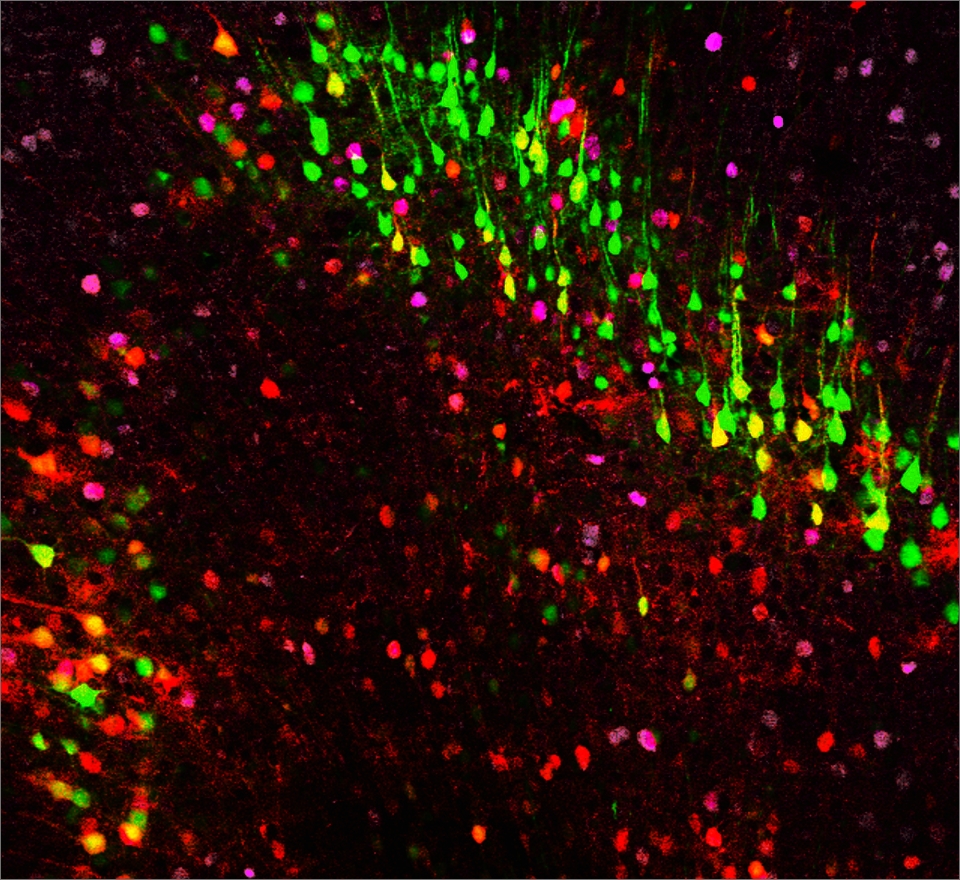
NeuExcell's in situ neuroregeneration technology developed from ATN ASTROCYTES TO NEURONS (TM) platform uses AAV as a vector to locally deliver neural transcription factors to specific regions. The neurons derived from astrocytes are the correct subtypes for the target region. This technology can be used to treat various neurodegenerative diseases including common and rare diseases.